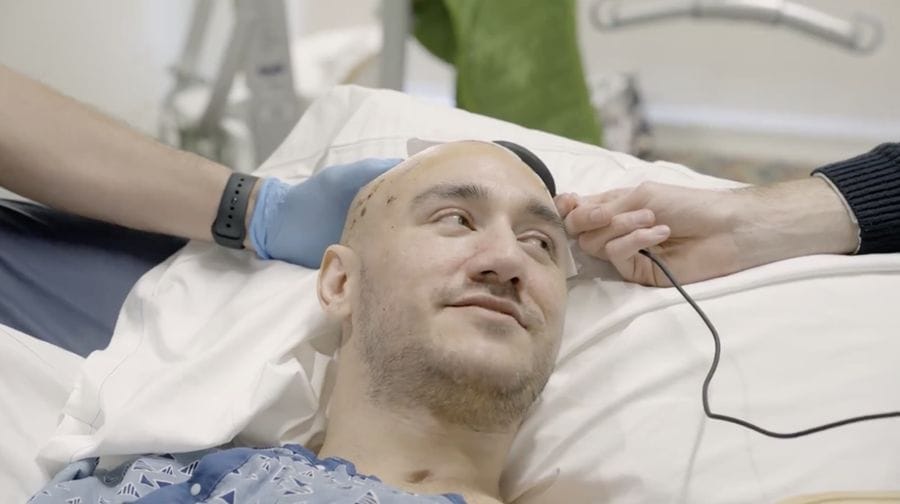
London — The frontier of neurotechnology is no longer theoretical. Elon Musk’s brain–computer interface company, Neuralink, has confirmed that 12 people worldwide have now received its experimental brain chip implants. The announcement marks a significant step forward in the company’s human trials, which began in 2024 after receiving long-delayed approval from the U.S. Food and Drug Administration.
According to Neuralink, patients—many of whom suffer from severe paralysis—are using the chip to control digital and physical devices purely through thought. Collectively, the group has logged more than 15,000 hours of usage across 2,000 days, demonstrating that the implants can function reliably over extended periods.
The technology is not without challenges. Neuralink admitted that in its first patient, a thin wire shifted position inside the brain, raising concerns about long-term stability. Still, the company insists that the device continues to perform within safety parameters.
Beyond the U.S., Neuralink is expanding trials to the United Kingdom, partnering with University College London Hospitals and Newcastle Hospitals. This international collaboration signals the company’s ambition to accelerate clinical validation and regulatory acceptance.
The project has attracted massive investment, with Neuralink securing US$650 million in funding as of June 2025. The capital is expected to support scaling trials, refining surgical techniques, and preparing for eventual commercialization.
For patients, the promise is profound: restoring communication, mobility, and independence to those with spinal cord injuries or neurodegenerative conditions. For society, the implications are even broader—raising questions about ethics, privacy, and the merging of human cognition with machines.
As the race to integrate brain–computer interfaces intensifies, Neuralink’s progress underscores both the transformative potential and the unresolved risks of this technology. What is clear is that the era of practical neurotech has already begun.